
As Antibody-Drug Conjugates (ADCs) continue to reshape the oncology therapeutic landscape, Litchlab officially announces the launch of LiLinker™, a dedicated linker development and CDMO platform designed to bridge molecular design with process-ready implementation. Engineered for clinical stability, controlled release, scalable conjugation, and global regulatory compatibility, LiLinker™ provides clients with end-to-end linker optimization, GMP-grade synthesis, analytical development, and IND/IMPD dossier preparation.
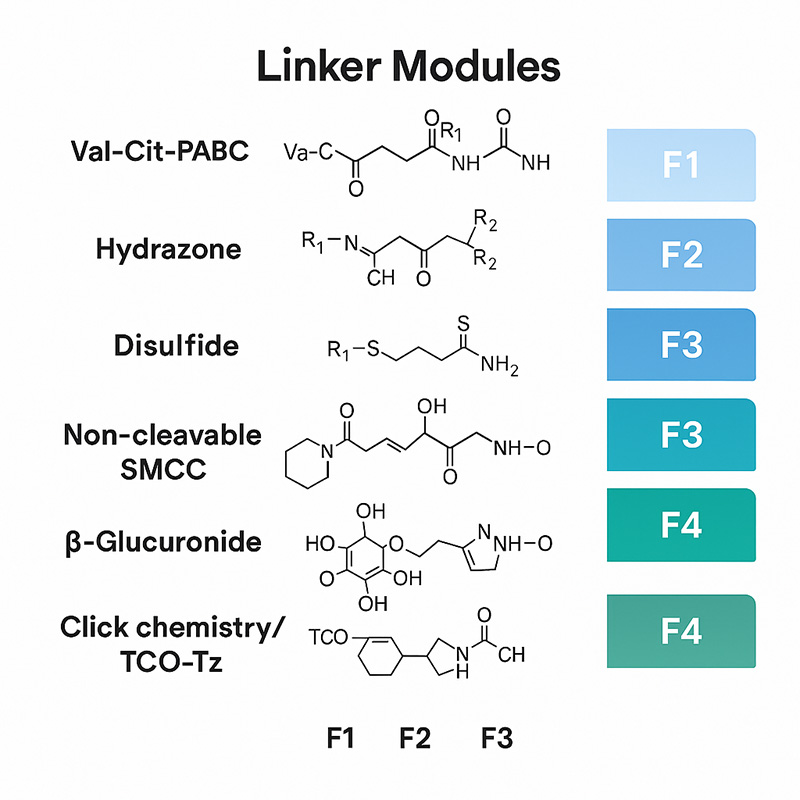
Litchlab’s LiLinker™ platform integrates six core chemical linker categories with four functional design classes to meet specific needs across diverse ADC pipelines:
| Linker Class | Representative Structure | Release Mechanism | Application Notes |
|---|---|---|---|
| Val-Cit-PABC | For MMAE/MMAF | Enzyme-cleavable | Gold standard in hematologic ADCs, fast lysosomal cleavage |
| Hydrazone | For DM1 or anthracyclines | pH-sensitive | Responsive in acidic TME but prone to plasma instability |
| Disulfide | For MMAF, SN-38 | Redox-sensitive | Rapid cleavage in high-GSH tumors; less stable in systemic circulation |
| SMCC (Non-cleavable) | For DM1, PBDs | Proteolytic degradation | Plasma-stable with residual payload on antibody |
| β-Glucuronide | For SN-38, Toxins | Enzyme-specific cleavage | Suited for solid tumors with high β-glucuronidase activity |
| Click Chemistry (TCO-Tz) | Dual payload designs | Bio-orthogonal | Ideal for dual-action ADCs or imaging-conjugates |
💡 Functional tiers (F1–F4) are used internally to match linker types with specific development goals:
F1 – Plasma stability enhanced
F2 – Site-specific conjugation compatible
F3 – Immune-stimulatory payloads supported
F4 – Dual-drug or synergistic payload delivery enabled
LiLinker™ is fully embedded into Litchlab’s broader bioconjugation infrastructure, offering:
| Service Module | Core Capabilities |
|---|---|
| Linker Screening & Selection | >25 proprietary and industry-standard linker formats, modeling of release kinetics & stability |
| Custom Synthesis | 0.5–50g scale linker manufacturing with impurity profile and residual solvent controls |
| Analytical Method Development | HPLC, LC-MS, UV, qNMR, residual linker quantification, DAR accuracy modeling |
| DAR Control Strategies | Conjugation conditions tuned to modulate drug-to-antibody ratio (DAR 2–8) via linker design |
| Stability Studies | pH/temperature-dependent linker stability across formulation matrices (pH 5.5/7.4 at 4–40°C) |
| ICH-Compliant Dossier Support | Q3A/B, Q6B, Q1A alignment, validation documentation for IND/IMPD submissions |
Litchlab’s linker development team supports full regulatory traceability, offering standalone CMC modules or integrated conjugation dossiers. Recent client engagements include:
| Project | Partner | Linker Type | Target | Region | Status |
|---|---|---|---|---|---|
| ADC-013 | US biotech | Val-Cit-PABC | Trop-2 | FDA | IND submitted, Module 3 prepared by Litchlab |
| ADC-047 | China pharma | SMCC (non-cleavable) | HER2 | NMPA | Process under GMP verification |
| ADC-101 | EU biotech | β-Glucuronide + DM1 | EGFR | EMA | Dual-cleavage linker in preclinical scale-up |
| ADC-X25 | Global dual-report | TCO-Tz + MMAF | Bispecific ADC | US + CN | Ongoing dual payload DAR control optimization |
LiLinker™ is fully interoperable with Litchlab’s core ADC development platform, enabling seamless transition between:
Payload design: Including hydrophilic/hydrophobic balance, release rate tuning
Conjugation route: Lysine, cysteine, or site-specific enzymatic pathways
Downstream formulation: Injectable liquid and lyophilized ADCs with validated linker retention profiles
All linkers are evaluated using in vitro release assays, serum stability, and forced degradation tests, supporting robust, scalable and submission-ready drug product development.
Litchlab is a platform-based CDMO focused on high-complexity drug delivery and ADC development, with integrated capabilities spanning:
Bioconjugation systems: Payload synthesis, linker development, site-specific conjugation
Delivery technolo
gies: Liposomes, LNP, albumin nanoparticles, co-loaded nanoformulations
Regulatory services: IND/IMPD documentation, eCTD formatting, QbD validation studies
Manufacturing capabilities: High-potency containment, GMP fill/finish, ADC formulation optimization
Visit: www.litchlab.com
Contact: RD1@litchlab.com