
Litchlab, an innovation-focused contract development and manufacturing organization (CDMO) specializing in complex drug delivery systems, has officially launched its next-generation liposome platform — SynerLipo™. Engineered for dual-drug co-encapsulation, coordinated release, and precision combination therapy, SynerLipo™ is designed to bridge the gap between pharmacologic synergy and synchronized in vivo delivery, providing end-to-end CDMO support from formulation design to IND filing.
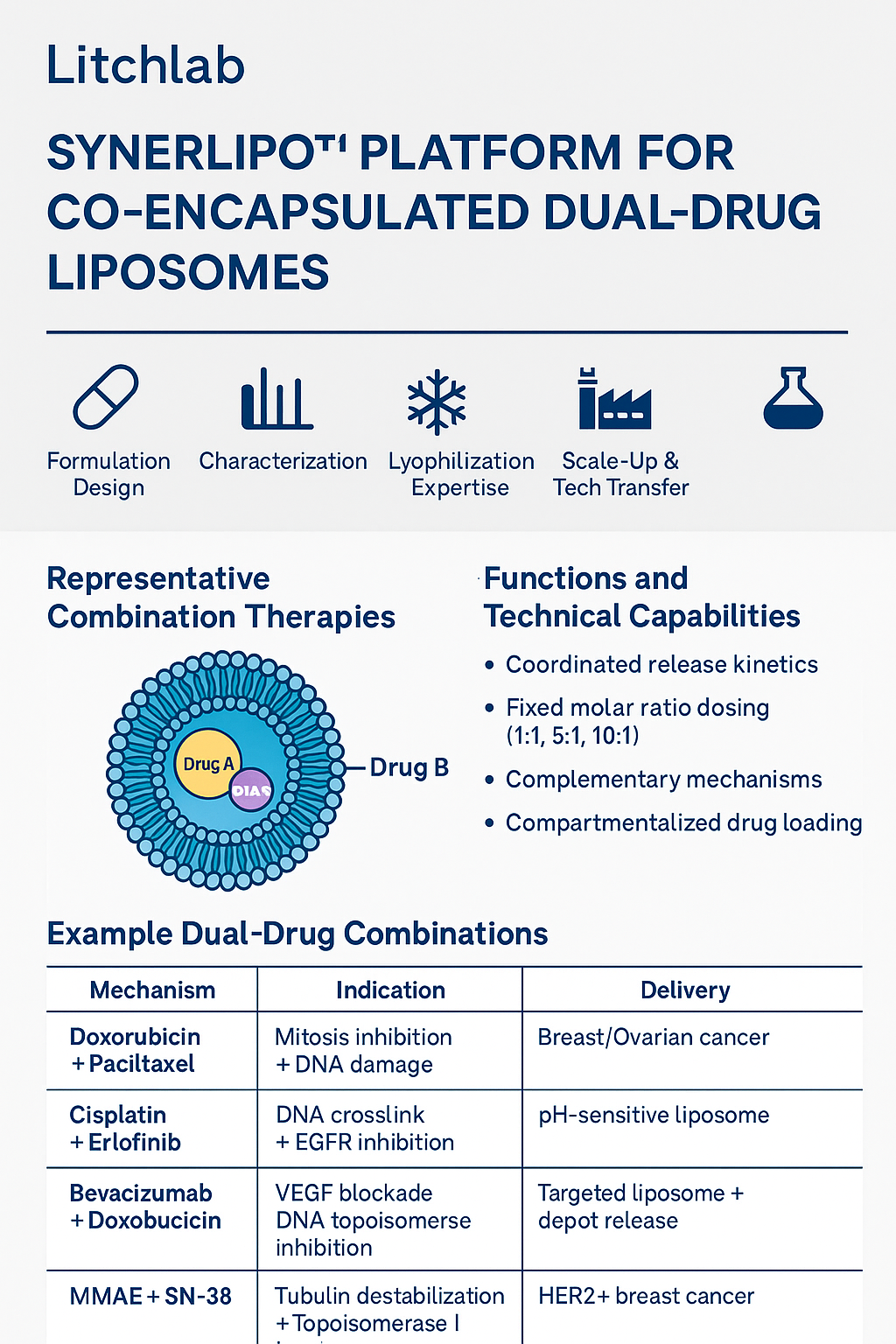
While combination therapy has become a mainstay of modern oncology, most regimens suffer from asynchronous exposure, poor pharmacokinetic matching, and limited tumor-targeting potential. The SynerLipo™ platform directly addresses these challenges by co-encapsulating two active agents into a single liposomal particle, delivering enhanced outcomes via:
🔹 Coordinated release kinetics through membrane/core spatial design
🔹 Fixed molar ratio dosing (1:1, 5:1, 10:1 and custom ratios) for optimal synergy
🔹 Mechanistic complementarity, combining cytotoxic, anti-angiogenic, immunologic or genetic modulators
🔹 Compartmentalized drug loading (hydrophilic/hydrophobic) for enhanced encapsulation and control
| Combination | Mechanism | Indication | Delivery Mode |
|---|---|---|---|
| Doxorubicin + Paclitaxel | DNA damage + microtubule disruption | Breast / Ovarian cancer | Lipid bilayer + aqueous core |
| Cisplatin + Erlotinib | DNA crosslinking + EGFR inhibition | NSCLC | pH-sensitive liposome |
| Bevacizumab + Doxorubicin | VEGF blockade + topoisomerase inhibition | Angiogenic solid tumors | Targeted liposome + depot release |
| MMAE + SN-38 | Tubulin inhibitor + Topo-I inhibitor | HER2+ breast cancer | ADC-like dual-drug nanocarrier |
| Paclitaxel + siMDR1 | Cytotoxic + MDR efflux suppression | Drug-resistant tumors | Nucleic acid-functionalized liposomes |
Litchlab’s SynerLipo™ platform integrates advanced formulation science, analytical development, and GMP readiness into a unified offering:
Compatible with dual hydrophobic/hydrophilic payloads
Loading techniques: pH-gradient, ammonium sulfate-driven remote loading, solvent injection, and ultrasonication
Custom lipid-drug conjugates for controlled release or improved stability
Synergy scoring: Chou-Talalay CI index, isobologram mapping
Dual-drug release kinetics modeling (zero-order, biphasic)
Cellular studies: cytotoxicity, apoptosis, cell cycle, resistance reversal
Microfluidic dual-channel control for fixed-ratio industrial loading
Freeze-drying process development (cryoprotectant screening, redispersion stability)
ICH Q1/Q6 stability testing (Zone I–IV, accelerated & real-time)
Full CMC package: raw data archiving, formulation/process reports, risk assessment
QbD framework: CPP and CQA definition, design space justification
Regulatory alignment for FDA, NMPA, EMA submissions
Litchlab is actively engaged in dual-drug liposome projects with biotechs and academic partners, including:
TNBC liposomal formulation for preclinical IND-track development with a North American biotech
Bevacizumab + Doxorubicin injectable liposome in partnership with a Southeast Asian oncology institute
Dual-drug freeze-dried kits for personalized oncology trials in Europe
We welcome global partnerships under flexible CDMO models including:
💼 Reformulation of existing APIs into long-circulating liposomes
💼 Development of fixed-ratio dual-drug formulations with IND-enabling data
💼 Controlled release or tumor-triggered formulations
💼 Scale-up from discovery to GMP batch production
Litchlab is a Shanghai-based technology-driven CDMO dedicated to the development of advanced drug delivery systems, including liposomes, LNPs, albumin nanoparticles, polymeric nanocarriers, and ADC linker-payloads. Our integrated services span formulation R&D, process transfer, analytical development, GMP batch production, and global regulatory documentation.
🔗 Website: www.litchlab.com
📩 Contact: RD1@litchlab.com