
With Antibody-Drug Conjugates (ADCs) becoming a cornerstone of targeted oncology therapies worldwide, Litchlab today announced the full deployment of its end-to-end ADC development and CDMO service platform. Leveraging its deep expertise in high-potency drug delivery systems (HDDS), conjugation chemistry, and complex injectable formulation, Litchlab now offers a fully integrated solution from payload-linker design to GMP manufacturing and IND submission support, positioning itself as a strategic partner for innovative biotech and pharmaceutical companies globally.
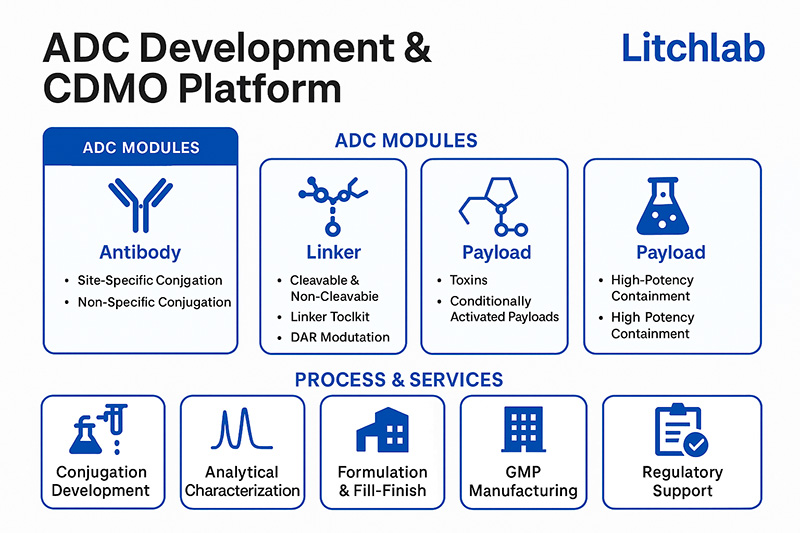
Litchlab’s ADC platform is built around the rational integration of three core components: Antibody, Linker, and Payload. It includes the following modular capabilities:
✅ Compatible with MMAE, MMAF, DM1, DM4, SN-38, duocarmycin, PBD, and more
✅ Category 4 containment facilities for HPAPI handling (OEL < 1 ng/m³)
✅ Development of cleavable or conditionally activated payloads (acid-sensitive, enzymatically cleavable, CYP450-activated systems)
✅ Cleavable linkers: Val-Cit, disulfide, hydrazone, β-glucuronide
✅ Non-cleavable linkers: SMCC, PEG-maleimide, triazole-based systems
✅ Internal linker toolkit supporting DAR modulation and controlled release kinetics
✅ Non-site-specific (Lys, Cys) and site-specific conjugation (Thiomab, engineered cysteine)
✅ Expertise in enzymatic, chemical, and microfluidic conjugation technologies
✅ DAR optimization protocols and platform screening for homogeneity and safety
To ensure product stability, reproducibility, and regulatory compliance, Litchlab provides advanced process development services including:
| Process | Capability |
|---|---|
| Conjugation Development | Controlled DAR profiles, optimized molar ratios, scalable protocols |
| High-Potency Containment | Isolated HVAC systems, validated CIP/SIP for toxic material cleaning |
| Analytical Characterization | HIC-HPLC, SEC, CE-SDS, LC-MS, iCIEF, MALS for DAR, aggregation, impurities |
| Formulation & Fill-Finish | Aseptic fill/lyo for liquid & lyophilized ADCs; buffer screening & stability |
Litchlab offers regulatory-aligned CMC services for NMPA, FDA, and EMA submissions, including:
eCTD-ready CTD Module 2 & 3 dossier preparation
Stability studies and forced degradation profiling
Comparative analytics and bridging studies for biosimilarity
Support for IND/IMPD filings, QbD justifications, and audit readiness
Litchlab is actively exploring next-generation ADC constructs, including:
Liposomal or polymeric nanoparticle-encapsulated ADCs for improved PK/PD and tumor penetration
Dual-Payload ADCs delivering cytotoxins and immune modulators simultaneously
ADC + Immunotherapy Combos with synergistic release of TLR agonists, STING agonists, or checkpoint inhibitors
These advanced formats aim to address limitations in tumor heterogeneity, systemic toxicity, and immune desert microenvironments.
🏭 ADC DS capacity: 1–10 L batch scale; aseptic containment-compliant
💉 Fill-finish: Up to 100,000 vials/year (liquid or lyophilized)
🤝 Global clients: Serving biotech, pharma, and research institutes across Asia, North America, and EU
🔍 Use Cases:
| Client Type | Project | Services |
|---|---|---|
| US/EU Biotech | IND-stage ADC | DAR optimization, linker design, preclinical CMC |
| China Pharma | Dual-payload ADC | Custom conjugation, scale-up, CMC dossier |
| Listed Pharma | Site-specific ADC | Advanced conjugation chemistry, global tech transfer |
“ADCs represent the convergence of biologics, small molecules, and chemical engineering. Our role is to enable that complexity to reach patients safely.”
— Dr. Yue Lu, Head of Delivery Platforms, Litchlab
📌 Website: www.litchlab.com
📩 Business Inquiry: RD1@litchlab.com