
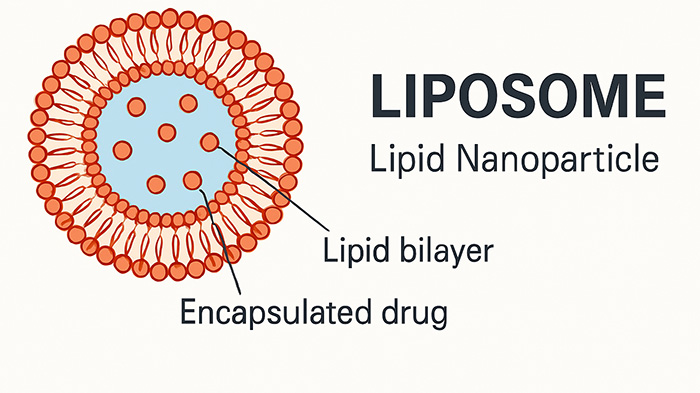
Litchlab is a specialized CDMO dedicated to the research, development, and production of liposome-based drug delivery systems. We offer comprehensive services that cover the entire drug development cycle — from early-stage formulation design to clinical batch production under GMP standards.
With proven expertise in liposomal technology, Litchlab delivers customized and robust solutions tailored to the physicochemical properties of your active pharmaceutical ingredients (APIs), ensuring enhanced bioavailability, optimized release profiles, and targeted delivery performance,
| Liposome Type | Key Features | Suitable APIs | Therapeutic Applications |
|---|---|---|---|
| Conventional Liposomes | Classic phospholipid bilayer; flexible for both hydrophilic and lipophilic drugs | Small molecules / peptides | Oncology, anti-infective therapies |
| PEGylated (Stealth) Liposomes | Surface PEGylation for extended circulation time and RES evasion | Small molecules / proteins | Tumor targeting, immune modulation |
| Cationic Liposomes | Positively charged surface enabling nucleic acid complexation | siRNA / mRNA / pDNA | Gene therapy, mRNA vaccines |
| pH-Sensitive Liposomes | Controlled release under acidic microenvironments | Small molecules / nucleic acids / peptides | Tumor microenvironment, inflammation sites |
| Thermo-Sensitive Liposomes | Temperature-triggered drug release for spatial control | Small molecules | Localized cancer treatment |
| Immunoliposomes | Surface-modified with antibodies or ligands for active targeting | Small molecules / peptides / proteins | Targeted oncology and immunotherapy |
Formulation Development
Lipid composition design based on API properties, targeting efficiency, release kinetics, and stability.
Nucleic acid-encapsulated cationic or ionizable lipid systems for mRNA, siRNA, and DNA.
Preformulation screening for encapsulation efficiency, particle size, and controlled release.
Process Development & Scale-up
Comprehensive liposome manufacturing techniques: thin-film hydration, ethanol injection, microfluidic-based methods.
Robust process transfer from laboratory to GMP production scale.
CPP/CQA definition and process optimization based on regulatory expectations.
Analytical Characterization
Particle size & PDI analysis (Dynamic Light Scattering, DLS).
Zeta potential measurement.
Encapsulation efficiency & drug loading (HPLC/UV/ELSD).
Leakage rate & in vitro release testing.
Morphology analysis (Cryo-TEM, AFM).
Stability studies under ICH guidelines.
Lyophilization Development
Cryoprotectant screening and lyophilization cycle optimization for enhanced stability.
Reconstitution behavior validation post-lyophilization.
Accelerated stability testing for shelf-life prediction.
1️⃣ Project Assessment & Feasibility Study
→ Liposome type selection based on API characteristics and intended therapeutic use.
2️⃣ Formulation Optimization & Small-Scale Prototyping
→ Rapid evaluation of encapsulation efficiency, particle size distribution, release profile, and stability.
3️⃣ Process Scale-up & GMP Transfer
→ Smooth transition from lab-scale process to GMP-grade manufacturing.
4️⃣ Analytical Method Development & Validation
→ ICH-compliant analytical method establishment and validation.
5️⃣ GMP Clinical Batch Production
→ cGMP-compliant manufacturing for clinical and commercial drug product supply.
✔️ Comprehensive portfolio of liposome types for various APIs and therapeutic areas.
✔️ Established end-to-end process — from formulation development to GMP batch manufacturing.
✔️ Advanced lyophilization platform for extended product stability and global logistics readiness.
✔️ Extensive experience in nucleic acid liposome formulations, including multiple IND-supporting projects.
✔️ Full regulatory support for IND, NDA, and ANDA submissions (FDA, EMA, NMPA).
📧 Contact: RD1@litchlab.com
📩 Learn more or inquire about a project: www.litchlab.com