
As the pharmaceutical industry moves toward increasingly complex therapeutic modalities—ranging from mRNA and gene editing to protein biologics and targeted small molecules—the demand for multi-compatible, customizable, and clinically translatable drug delivery platforms is growing rapidly. In response, Litchlab, a global contract development and manufacturing organization (CDMO), has launched a robust, multi-molecule nanocarrier platform designed to deliver a wide array of therapeutic payloads with precision, safety, and scalability.
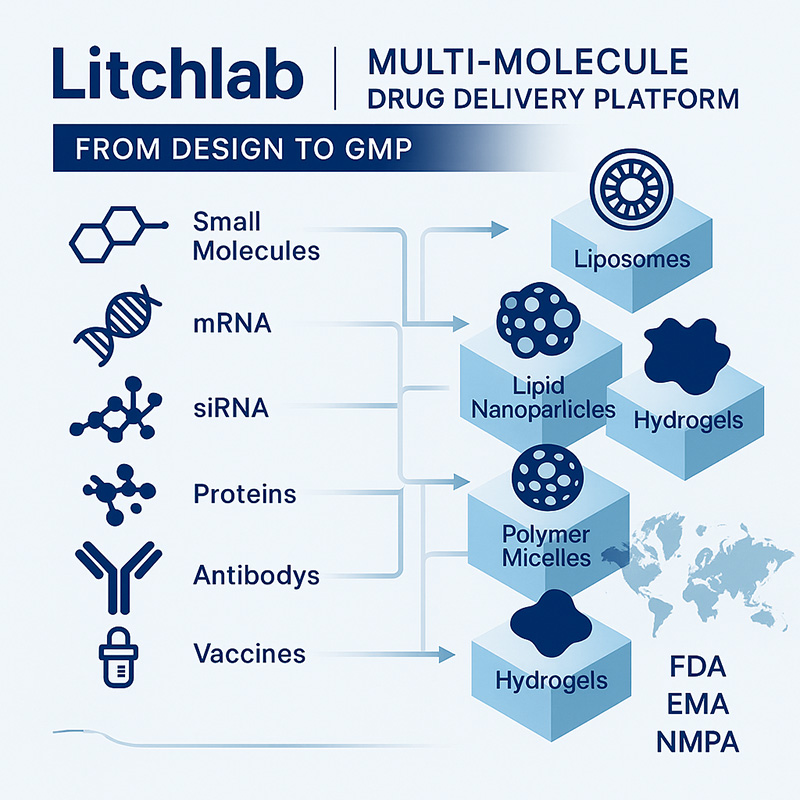
1. Broad Payload Compatibility Across Modalities
Litchlab's next-generation delivery systems support a wide variety of therapeutic classes, including:
Small molecules (e.g., chemotherapeutics, kinase inhibitors)
Nucleic acids (mRNA, siRNA, ASOs, CRISPR-Cas constructs)
Proteins and peptides (enzymes, therapeutic peptides, antibodies)
Antibody-based drugs (mAbs, bispecifics, ADC precursors)
Vaccines (subunit proteins, nucleic acid-based, peptide vaccines)
2. Technological Backbone: A Modular Nanocarrier Ecosystem
Lipid-based systems (LNPs, liposomes): Ideal for nucleic acid delivery and combination therapies
Polymer micelles and nanoparticles: Controlled release and tumor targeting
Albumin nanoparticles and mesoporous silica: Enhanced solubility and organ-specific delivery
Block copolymer carriers with click chemistry: Tunable targeting and intracellular transport
Magnetic nanoparticles and functional hydrogels: For image-guided therapy and tissue regeneration
3. Synergistic Delivery Applications
Cancer immunotherapy: Co-delivery of PD-L1 inhibitors and RNA therapeutics to remodel tumor immune microenvironments
Inflammatory disease: Dual loading of IL-10 proteins and anti-inflammatory agents for enhanced local efficacy
Vaccine enhancement: LNP-based delivery of mRNA antigens and immune adjuvants for potent and stable immunogenicity
Litchlab offers an integrated development pathway from early-stage screening to clinical manufacturing, including:
Formulation and delivery system optimization
Microfluidics- and flow-based scalable manufacturing
Stability optimization via lyophilization
GMP-ready pilot and clinical-scale production
Regulatory support for IND/IMPD (FDA, EMA, NMPA)
All platform systems are developed under a rigorous CMC framework, compliant with ICH Q8/Q9/Q10 standards, ensuring fast and compliant transitions from preclinical development to clinical and commercial phases.
With the rise of mRNA vaccines, protein-based therapies, and nucleic acid drugs, traditional drug delivery approaches are struggling to meet the evolving requirements of bioavailability, stability, tissue specificity, and immunocompatibility. Litchlab’s modular, multi-molecule-compatible systems are tailored to overcome these challenges.
According to Evaluate Pharma, by 2030, over 40% of global novel therapeutics will be biologics and nucleic acid-based molecules, creating an urgent need for platforms that support payload diversity, personalized design, and clinical scalability.
Litchlab is an international CDMO specializing in advanced drug delivery solutions for biologics, nucleic acids, small molecules, and antibody therapeutics. With comprehensive capabilities in formulation, process development, scalable manufacturing, and regulatory support, Litchlab serves biopharmaceutical companies across the U.S., EU, Japan, and China, and is committed to being a strategic partner in translational medicine.
Contact for Collaboration: sales@litchlab.com
Website: www.litchlab.com
🔍 Keywords: multi-molecule delivery system, LNP, CDMO, mRNA, targeted drug delivery, nucleic acid therapeutics, nanocarriers, protein therapeutics, ADC delivery, biologics formulation